CITI & IRB Training
It is the position of the Rice IRB that all personnel on IRB protocols require an appropriate basic human subjects research training course to ensure that research involving human subjects meets the strict ethical guidelines enshrined in the Belmont Report and the oversight of the Office for Human Research Protections at the US Department of Health and Human Services.
Principal Investigators and research personnel are required to complete the Collaborative Institutional Training Initiative (CITI) Human Subjects Research training course(s) prior to approval of their study:
Instructions for New Users
- Go to the CITI home page.
- Click “Register” in the upper right corner.
- In Step 1, choose Rice University (SSO) as your institution from the dropdown list.
- Read and review the Terms of Service and Privacy Policy for CITI, and when you are comfortable with them, check the boxes that you agree with them and to affirm that you are an affiliate of Rice University.
- On the next page, log in with your NetID and Password.
- Confirm your language preference, institutional email address, degree, employee or student ID number, department, role, address, and phone.
- Please use your Rice email address as one of your email addresses, if applicable. (A second, non-Rice email can be added in your profile.)
- Search the institutional courses available to Rice University. Click on the “Courses” button connected to Rice University. Click on “Learner Tools” and “Add a Course.”
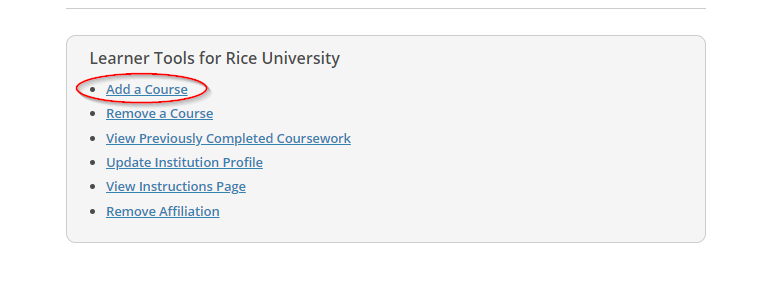
- For Question 1, please select “Human Subjects Research” to find Rice CITI courses.
- For Question 2, select the box(es) that most closely align with your research.
- For example:
i. If you are conducting behavioral research, please select “Social & Behavioral Research Investigators” and you will be assigned to complete the course “Social & Behavioral Research - Basic/Refresher,” which meets the training requirement for Social/Behavioral research at Rice University.
ii. If you are conducting research that involves taking samples (e.g., blood, tissue, saliva, DNA, etc.) directly from research participants, please select “Biomedical Research Investigators” and you will be assigned to complete the course “Biomedical Research – Basic/Refresher”.
iii. If you are conducting research that involves using samples (e.g., blood, tissue, saliva, DNA, etc.) that have already been collected from human research participants who you will not have contact with, please select “Research with data or laboratory specimens - ONLY” and you will be assigned to complete the course “Data or Specimens Only Research – Basic/Refresher”.
- Please note that it may be necessary or appropriate to select more than one training course!
- Per NIH policy, if you’re conducting NIH-funded research, please complete a GCP course that most closely aligns with your research. GCP training does not replace the standard human subject research training required by the IRB.

- For example:
Completion of this training by Rice-associated personnel will verified by an IRB Administrator at the time of the review of the study. Administrators can verify this training in CITI directly; you do not need to email your training certificates to the IRB or attach them to the protocol. For IRB personnel who are not associated with Rice, completion certificates should be uploaded.
You may print certificates of completion once all of the required modules have been completed successfully through the "Print" link on the learner’s menu. We recommend that PIs on the study consider keeping a copy of the study researchers’ training for their records.
CITI Course Renewal - Refresher Course
CITI certification is valid for 3 years. CITI will email you an automatic training reminder 60 days before your training expires and prompt you to complete a Refresher Course for recertification. Training will be verified during IRB Administrator review.
Returning CITI Users Who Are New to Rice University
- Go to the CITI home page.
- Log in using your username and password from your previous institution.
- Click on the link “Add Institutional Affiliation.”
- Choose Rice University as your institution.
- Fill in the required information on the next page.
- Make sure that your NetID is one of the email addresses associated with your account.
- Click “Next” to advance to the curriculum selection.
FAQ
- Does Rice track CITI training for all research personnel?
-
While we do not have a database to track training status, we do use CITI administrative access to verify training records for all personnel listed in a protocol when protocols are submitted for review. For this reason, it is unnecessary to email your training certificates to the IRB or attach them to the IRB protocol for Rice-associated personnel.
For personnel not associated with Rice, please attach training certificates to the IRB protocol.
- When do I need to complete CITI training?
-
Study personnel must complete all required CITI training prior to protocol approval, which includes new submissions, modifications, and renewals.
- How long is my CITI certificate valid?
-
The CITI certificate is valid for 3 years for Basic/Refresher training.
- How do I know when to renew my CITI certification?
-
CITI will email you an automatic training reminder 60 days before your training expires and prompt you to complete a Refresher Course.
- Do I need to take the GCP course(s)?
-
Per NIH policy effective January 1, 2017, all NIH-funded clinical investigators and clinical trial staff who are involved in the design, conduct, oversight, or management of clinical trials are required to be trained in Good Clinical Practice (GCP).
Rice’s CITI account currently provides the following GCP courses:
- Good Clinical Practice Course, US FDA Focus
- Good Clinical Practice Course for Clinical Trials Involving Medical Devices (international focus)
- Good Clinical Practice Course for Clinical Trials Involving Investigational Drugs (ICH / international focus)
- GCP – Social and Behavioral Research Best Practices for Clinical Research
GCP training does not replace the standard human subject research training required by the IRB.
- Does the Responsible Conduct of Research (RCR) course satisfy Rice’s requirement for human subjects research training?
-
No. RCR training covers research integrity and is a specific requirement for projects funded by the National Institutes of Health (NIH), National Science Foundation (NSF), and National Institute of Food and Agriculture (NIFA). Although these courses share some information, they are not interchangeable.
HSR training is required for all personnel on all studies.
- Do I need additional training to collect or receive protected health information (PHI)?
-
Yes. You will need to take the “Research and HIPAA Privacy” course. This is an optional module within the basic human subjects research course.
- My Sponsor requires me to take a specific CITI course that isn’t available in Rice’s listed CITI curriculum. What do I do?
-
Please reach out to an IRB Compliance Administrator for assistance.
Have Questions?
For questions regarding IRB training, please contact an IRB Compliance Administrator in the Office of Research Integrity by email or by calling 713-348-4820.
If you are having difficulty with the CITI training course, technical support is available at citisupport@med.miami.edu or 305-243-7970 (8am-5pm Eastern).
Request a Classroom or Lab Presentation
The Human Research Protection Program provides educational resources and training opportunities for those involved in human subjects research. Faculty can arrange for class or lab presentations to discuss ethics, the history of human subjects research and/or the application process. To schedule a presentation, please contact an IRB Compliance Administrator.
